

Overview
This protocol will walk you through the process of changing cell media for HUVECs. Medium changes essentially feed the cells and keep them healthy by providing fresh nutrients. Cell passaging, on the other hand, is used to maintain cells in exponential growth. Each cell line has idiosyncrasies, but given proper care and attention, most cell lines are easy to maintain and grow. Read on for the methods of changing cell media for HUVECs.
Materials
- Human Umbilical Vein Endothelial Cell Flasks
- Endothelial Growth Medium-2 (EGM™-2)
- Micropipettes
- Micropipette Tips
- Microscope
- Incubator (37˚C, 5% CO2, 90% humidity)
- Timer
- 70% Ethanol
- Kimwipes
- Vacuum Bottle
Methods
- Warm EGM™-2 to 37˚C in the water/bead bath;
- Spray it down with ethanol and place them inside a biosafety cabinet (BSC);
- Spray down incubator doors with ethanol and minimize breathing to diminish contamination risk;
- Remove cell culture flasks from incubator and place them under microscope to ensure there is no contamination;
- Still, under the microscope, check for flask confluency by analyzing the ratio of surface area occupied by cells and surface area not occupied by cells. It is good practice to passage cells that are ~90% confluent to ensure a high yield. If confluency is ~70-85%, follow this protocol on cell passaging;
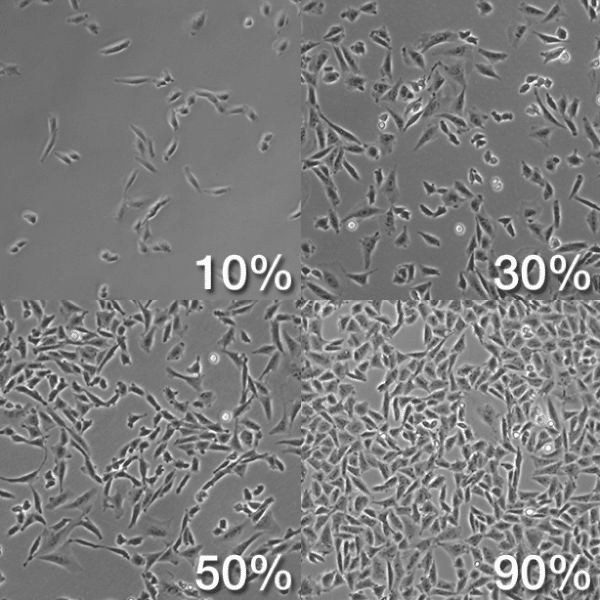
https://biology.stackexchange.com/questions/29857/origin-of-term-confluency-in-cell-culture - Spray down flasks with ethanol, wipe them with kimwipes and place them in BSC;
- Aspirate cell media from culture flasks, ensuring to avoid touching the bottom of the flask;
- Add fresh cell medium to your flasks;
- Note: if your cells are 25-45% confluent, add 0.3 mL/cm2. If your cells are 45-70% confluent, add 0.4 mL/cm2;
- Place your flasks back into the incubator;
- Feed every 48 hours.
For more protocols on how to change cell media, passage, freeze, and thaw cells – click here.

