
- About Allevi
- Bioprinters
- BioinksAdditivesAdditivesBioinksAdditivesAdditivesAdditivesAdditivesAdditivesAdditives
- Software
- Services
- Resources
- Support
Menu
Thermoplastics in bioprinting are often used as a support structure to provide extra mechanical strength and as reinforcement for matrix bioinks[1,2]. Polycaprolactone (PCL) is a biodegradable thermoplastic used in a variety of medical applications, including bioprinting. This study tested the viability and print resolution of PCL with the Allevi 2.
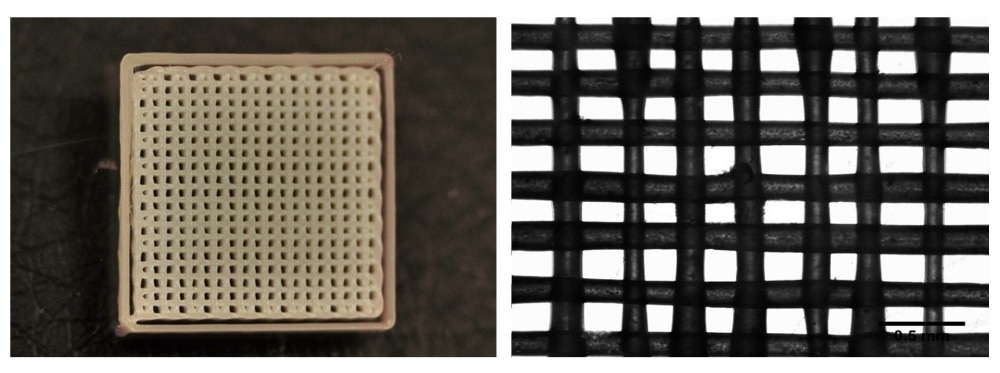
PCL was printed using print parameters and settings provided by Allevi (Figure 1). Line width and pore size were analyzed with ImageJ software. Five measurements were taken of 3 separate prints of the same design (Figure 2). Pore width average was 140 ± 38 μm, while line width average was 150 ± 38 μm.
Primary Human Neonatal Dermal Fibroblasts (HNDFs) were cultured at 37°C and 5% of CO2. HNDFs were cultured using Dulbecco’s Modified Eagle Medium supplemented with 10% fetal bovine serum, and 1% penicillin-streptomycin-amphotericin. Passage numbers under 10 were used.
Viability of PCL was tested with cell seeding of human neonatal dermal fibroblasts. First, PCL was sterilized through an overnight wash in 100% ethanol. Then, thin films were fabricated by melting 0.023 g of PCL at 90°C on a glass coverslip, then flattening with a second glass coverslip. Thin films were then allowed to cool to room temperature before removal from coverslips. Films were then cut to fit into 96 well plates.
HNDFs were suspended at a concentration of 250,000 cells mL-1 and pipetted into the wells containing the PCL thin films. Enough cell solution was used to submerge the samples. After overnight incubation, media was exchanged. Films were suspended in a cell concentration of 250,000 cells/ml.
To quantitatively assess cell viability, PrestoBlue Assay (Promega) was performed on days 1,3 and 7 of culture according to manufacturer’s protocol and analyzed using a BioTek Synergy 2 Plate Reader.
Statistical analysis was performed using an Analysis of Variance (ANOVA) test to determine if differences were present amongst treatment groups. If differences were determined from the ANOVA, a post hoc Tukey’s multiple comparison test was used to determine statistical differences between groups tested. A confidence level of 95% (α=0.05) was used for all analyses. Error bars on graphs represent the standard deviation from the mean.
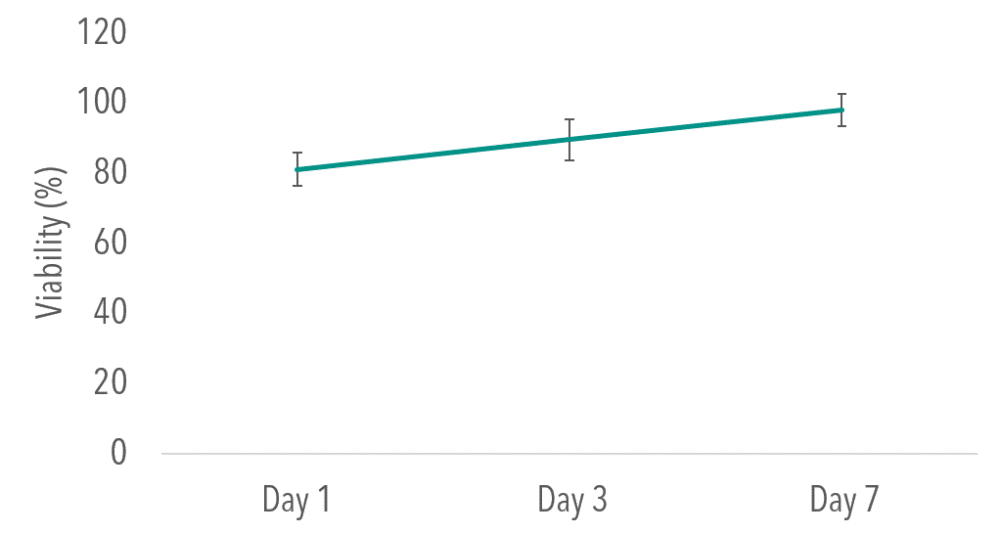
To determine viability of cells seeded on PCL, a PrestoBlue assay was used to quantitatively measure and compare viability to a 2D culture control. When compared to 2D controls, no significant differences were found at Day 1,3 or 7 time points (P> 0.05). Figure 3 depicts viability results for thin films normalized to 2D control.
These results demonstrate the ability of PCL to support viable cultures and create consistent, high resolution 3D geometries. This support material can be used in combination with matrix bioinks or on its own to create 3D culture environments for tissue engineering.
[1] J. Kundu and e. al, “An Additive Manufacturing-Based PCL-Alginate-Chondrocyte Bioprinted Scaffold for Cartilage Tissue Engineering,” Tissue Engineering: Part B, vol. 14, no. 2, 2008.
[2] J.-H. Shim et al, “Bioprinting of a Mechanically Enhanced Three-Dimensional Dual Cell-laden Construct for Osteochondral Tissue Engineering Using a Multi-head Tissue/Organ Building System,” Journal of Micromechanics and Microengineering, vol. 22, no. 8, July 2012.