
Just as a 2D printer has a variety of color inks to create an assortment of images, bioprinters utilize a variety of bioinks to create an assortment of viable 3D environments. One of the first steps in the bioprinting process involves choosing what bioinks to print. Read on for the full Allevi guide to matrix bioinks.
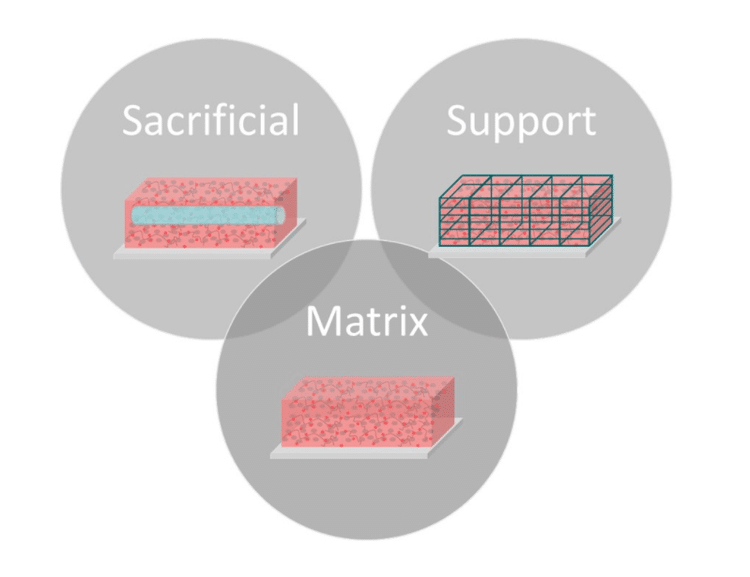
Bioinks for extrusion bioprinters can be split into three general categories: matrix, sacrificial, and support. Matrix bioinks include any biomaterial used for cell encapsulation. In addition to printability, these materials must offer a compatible environment for living cells. Most matrix bioinks are naturally derived hydrogels, such as collagen, hyaluronic acid or alginate [Table 1]. A hydrogel is a very hydrated material of hydrophilic polymers that crosslink to form 3D networks[1]. These materials offer the necessary permeability for nutrient and waste transport as well as a non-toxic gelation process.
| Matrix Bioink | Source | Gelation Time | Gelatin Process | Support/Sacrificial needed? | Sources |
|---|---|---|---|---|---|
| Poly(ethylene glycol) diacrylate (PEGDA) | Synthetic | Minutes | Chemical | No | (1-8) |
| Methacrylate Chondroitin Sulfate | Natural | Minutes | Chemical | No | (6,9) |
| Sodium Alginate | Natural | Seconds | Chemical | Yes | (6,10-14) |
| Cellulose (various modifications) | Natural | Minutes | Chemical | No | (15-18) |
| Gellan Gum | Natural | Seconds | Chemical | No | (19-21) |
| Fibrin | Natural | Seconds | Chemical | Yes | (21-25) |
| Cell- and Tissue- derived ECM (ie, Matrigel) | Natural | Minutes | Thermal | No | (26-29) |
| Collagen | Natural | 0.5 – 1h | Thermal | Yes | (29-31) |
| Spider Silk | Natural | Modification Dependent | Modification Dependent | Modification Dependent | (32) |
| Hyaluronic Acid (various modifications) | Natural | Modification Dependent | Modification Dependent | Modification Dependent | (6,9,30,33-36) |
| Dextran (various modifications) | Natural | Modification Dependent | Modification Dependent | Modification Dependent | (34,37) |
| Gelatin (various modifications) | Natural | Modification Dependent | Modification Dependent | Modification Dependent | (24-25, 38-42) |
If matrix materials have too long of a gelation time or lack the mechanical stability for printing on their own, support or sacrificial bioinks may be used to provide the necessary structure needed to create complex geometries. Some common matrix bioinks are listed in Table 1. These bioinks can be used on their own or combined together to create hybrid inks.
Important Bioink Properties
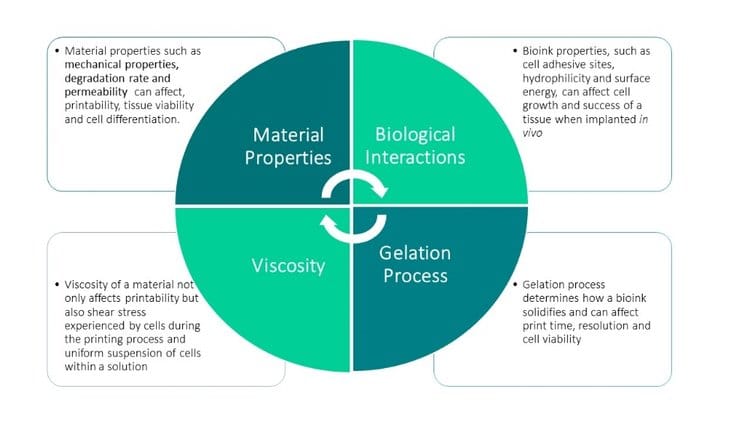
With so many options, choosing the best matrix bioink for specific applications can be daunting. While different applications and cell types will require different bioinks, there are a few general properties that are important to consider when choosing a bioink, including viscosity, gelation process, biological interaction, and general material properties.
Viscosity
In general, the rheological properties and polymer structure are very important when considering any bioink to ensure smooth, consistent extrusion. Specifically, matrix bioinks must work in multiple phases, first as a fluid during cell encapsulation, then as a solid once dispensed out of the printer. This transition generally works best with shear-thinning materials.
A shear-thinning material is a non-newtonian material that exhibits a decrease in viscosity as shear rate increases. This phenomenon is caused by the reorganization of polymer chains due to the shear rate, which leads to decreased entanglement and thus lower viscosity [43]. This property minimizes shear stress on cells and promotes physical gelation as viscosity increases sharply right after extrusion [43,44].
Gelation Process
The gelation process, or how a matrix bioink forms a solid structure after extrusion, can affect both viability and print resolution. Ideally, the gelation process for matrix bioinks should be fast as well as non-toxic to cells. Bioinks undergo either a physical or chemical gelation process, and these processes may be reversible or irreversible [44]. Some common gelation processes are described in more detail below. Researchers will use one of these individual methods or, especially when working with hybrid bioinks, may combine gelation methods together [24,25].
| Mechanism | Advantages | Disadvantages | Examples | Sources |
|---|---|---|---|---|
| Ionic | Constant viscosity during print due to reversible interactions | Post-process crosslinking needed with additional crosslinking agent Mechanically weak construct | Alginate | (6,10-14,17) |
| Stereocomplex | Constant viscosity during print due to reversible interactions | High viscosity of building blocks, needs post-process crosslinking, mechanically weak constructs | Stereocomplexed PEG-PLA block copolymers, Dextran stereocomplexed hydrogels | (43,45) |
| Thermal | Gelation process simple and highly compatible with biological systems | Long gelation time | Matrigel, Collagen, Agarose | (26-29) |
| Photocrosslinking | Photopolymerization after extrusion does not affect viscosity during extrusion; Post-process crosslinking for tunable mechanical properties | Exposure to free radicals may affect viability | Gelatin Methacrylate;Poly(ethylene glycol) diacrylate; Collagen Methacrylate | (1-9,36,41) |
| Enzymatic | Highly compatible with cells | Gelation can be too fast, need special extruder or post-crosslinking to avoid gelation prior to extrusion; low mechanical properties | Fibrin | (21-25) |
| Click Chemistry (Michael-type Addition) | Tunable mechanical properties | Long gelation time | Modified multi-arm poly(ethyelene glycol) | (46-47) |
Ionic Crosslinking
Ionic crosslinking creates physical crosslinks through cation solutions. Sodium alginate, a common matrix bioink, gels with Ca2+ ions. This process is compatible with cells and the degree of gelation can be tuned through the adjustment of CaCl2 concentration in solution [43–44]. While it offers constant viscosity during prints due to reversible interactions, the drawbacks include the need for post-process crosslinking with an additional crosslinking agent and mechanically weak constructs [44].
Stereocomplex Crosslinking
Stereocomplex crosslinking involves the coupling of lactic acid oligomers of opposing chirality [43]. These oligomers can be coupled with water-soluble polymers such as dextran or polyethylene glycol to form solid hydrogels [43,45]. Additional forms of complexation mechanisms include the formation of inclusion complexes such as cyclodextrins. In these cases, hydrophilic polymers are derivatized with cyclodextrin units and combined with guest molecule-derivatized polymers to create a hydrogel [43]. This mechanism offers constant viscosity during printing due to reversible interactions but requires post-process crosslinking and leads to mechanically weak constructs [43].
Thermal Crosslinking
Thermal crosslinking involves materials that gel from temperature changes. For matrix bioinks, thermal crosslinking must occur at room or body temperature (25 – 37 °C). While this process requires longer gelation times, the simple mechanism for gelation is highly compatible with biological systems.
Photocrosslinking
Photocrosslinking gelation occurs through the process of free radical polymerization. First, matrix bioinks such as gelatin, poly(ethylene glycol) and collagen are modified with acrylate groups. Then, UV, blue or visible wavelength light excites free radicals from a curing bioink that serves as the photoinitiator, which interacts with a matrix bioink to create a solid gel through covalent bonds. As photopolymerization involves exposure to free radicals and sometimes harmful wavelengths, the length of crosslinking time must be minimized to avoid effects on viability. This polymerization process, which occurs after extrusion, does not affect viscosity during extrusion and allows for tunable mechanical properties through post-process crosslinking.
Enzymatic Gelation
Another method of gelation involves enzymatic crosslinking, such as the gelation of fibrin. These materials are gelled through an enzymatic reaction, such as the interaction of thrombin with fibrinogen to create fibrin gels. This process is highly compatible with cells, but you will need special extruders or a post-crosslinking process to avoid gelation prior to extrusion.
Click Chemistry (Michael-type Addition)
Through click chemistry, Michael-type addition reactions create a hydrogel through di-thiolated oligopeptides and vinyl sulfone groups on PEG[46]. This process offers tunable mechanical properties but has a long gelation time [47].
Support Material needed?
In cases where a matrix bioink takes a long time to gel or lacks in mechanical properties, support or sacrificial bioinks may be used in combination to enhance the final structure. Print methods, such as the FRESH method, can also help improve printability with matrix bioinks [48].
Biological Interactions
Physical properties of matrix bioinks, such as hydrophilicity and surface energy, can affect cell behavior, while materials with cell adhesive sites can enhance survival and proliferation of various cell types [44]. These biological interactions of materials can affect cell behavior and final outcome of a construct, so it is important to keep these properties in mind when choosing a matrix bioink [44]. Depending on specific application and cell type, different biological interactions may be desired [44].
The choice between natural or synthetic matrix bioinks can have a significant effect on biological interactions. To mimic native environments, many focus on natural polymers [44]. Often these materials also circumvent harsh fabrication conditions such as extreme temperatures and organic solvents. However, naturally derived bioinks cause challenges such as batch-to-batch variability, affecting rheological properties and reproducibility in prints, and poor mechanical properties [44]. Synthetic polymers solve the challenges of batch-to-batch variability and allow for control over degradation rates and functionalization, but do not offer the natural cell adhesive sites in many natural materials [44].
Material Properties
Other important matrix bioink properties to keep in mind include mechanical properties, degradation, permeability and hydration of a material. Different mechanical properties are desired for different cell and tissue types and can have a significant effect on cell growth and differentiation. Degradation properties are important to consider for biocompatibility and rate. Hydration and permeability of a material can affect material viscosity, oxygen and nutrient transport to cells, mechanical strength and the swelling or shrinking of a construct post-print [44].
As the direct materials used to encapsulate cells, matrix bioinks are one of the most crucial pieces of the bioprinting process. Each matrix bioink currently available offers both advantages and disadvantages. As bioprinting technologies continue to grow and become more relevant, more complex and specialized matrix bioinks will be developed. As opposed to one comprehensive bioink to solve current limitations, these future bioinks will cater to specific cells and tissues, allowing for the development of more accurate tissues and 3D environments.
References
[1] Z. Wang et al, “A Simple and High-Resolution Stereolithography-Based 3D Bioprinting System Using Visible Light Crosslinkable Bioinks,” Biofabrication, vol. 7, 2015.,
[2] S. Jana and A. Lerman, “Bioprinting a Cardiac Valve,” Biotech Adv, vol. 33, no. 8, pp. 1503-1521, 2015.
[3] R. F. Pereira and P. J. Bartolo, “3D Bioprinting of Photocrosslinkable Hydrogel Constructs,” J. Appl. Polym. Sci., vol. 132, no. 48, 2015.
[4] C. C. Hribar, P. Soman, J. Warner, P. Chung and S. Chen, “Light-assisted Direct-Write of 3D Functional Biomaterials,” Lab Chip, vol. 14, pp. 268-275, 2014.
[5] T. Q. Huang et. al, “3D Printing of Biomimetic Microstructures for Cancer Cell Migration,” Biomed Microdevices., vol. 16, no. 1, pp. 127-132, Feb 2014.
[6] G. D. Nicodemus and S. J. Bryant, “Cell Encapsulation in Biodegradable Hydrogels for Tissue Engineering Applications,” Engineering: Part B, vol. 14, no. 2, 2008.
[7] P. Soman et al, “Cancer Cell Migration within 3D Layer-by-Layer Microfabricated Photocrosslinked PEG Scaffolds with Tunable Stiffness,” Biomaterials, vol. 33, pp. 7064-7070, 2012.
[8] B. D. Fairbanks et al, “Photoinitiated Polymerization of PEG-diacrylate with lithium phenyl-2,4,6-trimethylbenzoylphosphinate: polymerization rate and cytocompatibility,” Biomaterials, vol. 30, no. 35, pp. 6702-6707, Dec 2009.
[9] M. Kesti et al, “A Versatile Bioink for Three-Dimensional Printing of Cellular Scaffolds Based on Thermally and Photo-triggered Tandem Gelation,,” Acta Biomaterialia, vol. 11, pp. 162-172, 2015.
[10] B. Duan et al, “3D Bioprinting of Heterogeneous Aortic Valve Conduits with Alginate/Gelatin Hydrogels,,” J Biomed Mater Res A., vol. 101, no. 5, pp. 1255-1264, June 2013.
[11] S. Khalil et al., “Bioprinting Endothelial Cells with Alginate for 3D Tissue Constructs,” J Biomech Eng, vol. 131, no. 11, October 2009.
[12] J. Kundu et al, “An Additive Manufacturing-Based PCL-Alginate-Chondrocyte Bioprinted Scaffold for Cartilage Tissue Engineering,” Tissue Engineering: Part B, vol. 14, no. 2, 2008.
[13] M. T. e. a. Poldervaart, “Sustained Release of BMP-2 in Bioprinted Alginate for Osteogenicity in Mice and Rats,” Plos one, vol. 8, no. 8, August 2013.
[14] S. SJ et. al, “Sodium Alginate Hydrogel-Based Bioprinting Using a Novel Multinozzle Bioprinting System,” Artif Organs, vol. 35, no. 11, pp. 1132-6, 2011.
[15] Q. Zhang et al, “Review on Biomedical and Bioengineering Applications of Cellulose Sulfate,” Carbohydrate Polymers., pp. 311-322, November 2015.
[16] B. Mohite et al, “A Novel Biomaterial: Bacterial Cellulose and its New Era Applications,” Biotechnology and Applied Biochemistry, vol. 61, no. 2, pp. 101-110, 2014.
[17] M. Kajsa et al., “3D Bioprinting Human Chondrocytes with Nanocellulose-Alginate Bioink for Cartilage Tissue Engineering Applications,” Biomacromolecules, vol. 16, no. 5, 2015.
[18] R. Adam et al, “3D Bioprinting of Carboxymethylated-Periodate Oxidized Nanocellulose Constructs for Wound Dressing,” Biomed Research International, 2015.
[19] R. Levato et al, “Biofabrication of Tissue Constructs by 3D Printing of Cell-laden Microcarriers,” Biofabrication, vol. 6, 2014.
[20] J. Visser et al, “Biofabrication of Multi-material Anatomically Shaped Tissue Constructs,” Biofabrication, vol. 5, 2013.
[21] D. Kirchmajer and e. al, “An Overview of the Suitability of Hydrogel-Forming Polymers for Extrusion-Based 3D-Printing,” J. Mater. Chem. B., vol. 3, pp. 4105-4117, 2015.
[22] Y.-B. Lee, “Bioprinting of collagen and VEGF-releasing Fibrin Gel Scaffolds for Neural Stem Cell Culture,” Experimental Neurology, vol. 223, pp. 645-652, 2010.
[23] T. A. Ahmed et al, “Fibrin: A Versatile Scaffold for TIssue Engineering Applications,” Tissue Engineering: Part B, vol. 14, no. 2, 2008.
[24] H.-W. Kang et. al, “A 3D Bioprinting System to Produce Human-Tissue Constructs with Structural Integrity,” Nature Biotechnology, vol. 34, no. 3, pp. 313-322, March 2016.
[25] D. B. Kolesky et al, “Three-Dimensional Bioprinting of Thick Vascularized Tissues,” PNAS Early Edition, 2016.
[26] S. Hong et al, “Cellular Behavior in Micropatterned Hydrogels by Bioprinting System Depended on the Cell Types and Cellular Interaction,” Journal of Bioscience and Bioengineering, vol. 116, no. 2, pp. 224-230, August 2013.
[27] L. Horvarth et al, “Engineering an in vitro Air-blood Barrier by 3D Bioprinting,” Nature Scientific Reports, vol. 5, 2015.
[28] D. W. G. Astashkina et al, “Critical Analysis of 3-D Organoid in vitro Cell Culture Models for High-Throughput Drug Candidate Toxicity Assessments,” Advanced Drug Delivery Reviews, vol. 69, pp. 1-18, 2014.
[29] A. D. Nocera et al., “Printing Collagen 3D Structures,” in VI Latin American Congress on Biomedical Engineering CLAIB 2014 (Vol 49), Parana, Argentina, IFMBE Proceedings, 2014, pp. 136-139.
[30] J. Y. Park et. al, “A Comparative Study on Collagen Type I and Hyaluronic Acid Dependent Cell Behavior for Osteochondral Tissue Bioprinting,” Biofabrication, vol. 6, 2014.
[31] V. Lee et al, “Design and Fabrication of Human Skin by Three-Dimensional Bioprinting,” Tissue Engineering: Part C, vol. 20, no. 6, 2014.
[32] K. Schacht et al, “Biofabrication of Cell-Loaded 3D Spider Silk Constructs,” Angew. Cehm. Int. Ed., vol. 54, pp. 1-6, 2015.
[33] C. B. Highley et al, “Direct 3D Printing of Shear-Thinning Hydrogels into Self-Healing Hydrogels,” Adv Mater, vol. 27, pp. 5075-5079, 2015.
[34] L. Pescosolido et al, “Hyaluronic Acid and Dextran-Based Semi-IPN Hydorgels as Biomaterials for Bioprinting,” Biomacromolecules, vol. 12, no. 5, pp. 1831-1838, 2011.
[35] C. B. Rodell, J. Mealy and J. A. Burdick, “Supramolecular Guest-Host Interactions for the Preparation of Biomedical Materials,” Bioconjugate Chem, 2015.
[36] J. A. Burdick et al, “Controlled Degradation and Mechanical Behavior of Photopolymerized Hyaluronic Acid Networks,” Biomacromolecules, vol. 6, no. 1, pp. 386-391, 2005.
[37] S. Levesque et al., “Macroporous interconnected Dextran Scaffolds of Controlled Pororsity for Tissue-Engineering Applications,” Biomaterials, vol. 26, pp. 7436-46, 2005.
[38] H. Aubin et al, “Directed 3D Cell Alignment and Elongation in Microengineered Hydrogels,” Biomaterials, vol. 31, no. 27, pp. 6941-6951, September 2010.
[39] J. W. Nichol et al, “Cell-laden Microengineered Gelatin Methacrylate Hydrogels,” Biomaterials, vol. 31, pp. 5536-5544, 2010.
[40] M. Nikkhah et al, “Directed Endothelial Cell Morphogenesis in Micropatterned Gelatin Methacrylate Hydrogels,” Biomaterials, vol. 33, no. 35, pp. 9009-9018, December 2012.
[41] L. Bertassoni et al, “Direct-write Bioprinting of Cell-laden Methacrylated Gelatin Hydrogels,” Biofabrication, 2014.
[42] L. E. Bertassoni and e. al, “Hydrogel Bioprinted Microchannel Networks for Vascularization of Tissue Engineering Constructs,” Lab Chip, vol. 14, pp. 2202-2211, 2014.
[43]J. Malda and e. al, “25th Anniversary Article: Engineering Hydrogels for Biofabrication,” Adv. Mater., vol. 25, pp. 5011-5028, 2013.
[44]J. K. e. a. Carrow, “Polymers for Bioprinting,” in Essentials of 3D Biofabrication and Translation, Elsevier Inc, January 2015, pp. 229-248.
[45]K. Boere, “Hybrid Dual Cross-linked Hydrogels: Injectable and 3D-Printable Biomaterials,” Utrecht Institute for Pharmaceutical Sciences, Utrecht, the Netherlands, 2015.
[46]D. Seliktar, “Designing Cell-Compatible Hydrogels for Biomedical Applications,” Science, vol. 336, pp. 1124-1128, 2012.
[47]A. Shikanov et. al, “Characterization of the Crosslinking Kinetics of Mult-arm Poly(ethylene glycoL) Hydrogel formed via Michael-type Addition,” Soft Matter, vol. 12, pp. 2076-2085, 2016.
[48]T. J. Hinton et al., “Three-dimensional Printing of Complex Biological Structures by Freeform Reversible Embedding of Suspended Hydrogels,” Science Advances, vol. 1, no. 9, 23 Oct 2015.

