
Nothing is quite as frustrating as an unexpected loss of viability when working with 3D cultures. Below is a troubleshooting guide that outlines the most common variables affecting viability in 3D cultures and what steps to take to create viable, healthy 3D bioprinted cultures.
General 3D Culture Variables
Before focusing on parameters in the 3D printing process that can affect viability, let’s start with parameters that affect viability in 3D cultures in general. You can test if these parameters are affecting your culture viability through an encapsulation study. We suggest completing an encapsulation study (or studies) to first characterize these parameters before advancing to bioprinted cultures, which will add more variables.
Cell Culture Contamination
A 2D control in all experiments is always useful. If this control demonstrates low viability, there is likely an issue with your initial cell cultures. (Issues with contamination? We love this useful guide for troubleshooting general cell culture contamination.)
Material contamination or toxicity
Are you working with a new material, or is there any potential for contamination of your material during preparation of 3D cultures? It is always a good idea to have a pipetted thin film control to assess for any potential issues with your material.
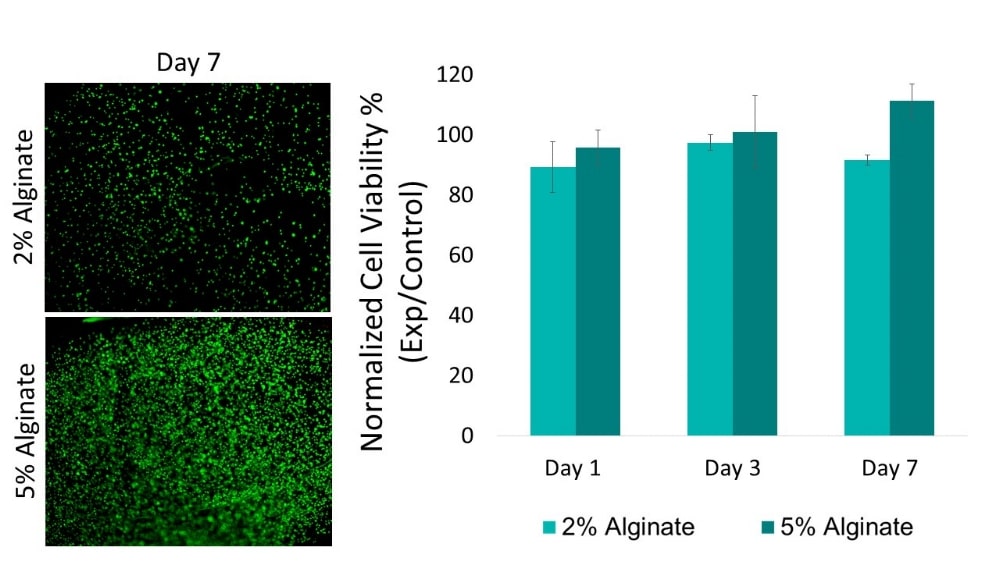
Cell Concentration
High cell density can initially maintain viability and proliferation but may lead to hyperplasia or apoptosis, while low cell density may result in low proliferation leading to decreased viability. Cell type and material permeability can affect necessary cell concentration needed to maintain high viability. We suggest running an encapsulation study to test varying cell concentrations whenever using a new cell type, material or crosslinking parameter.
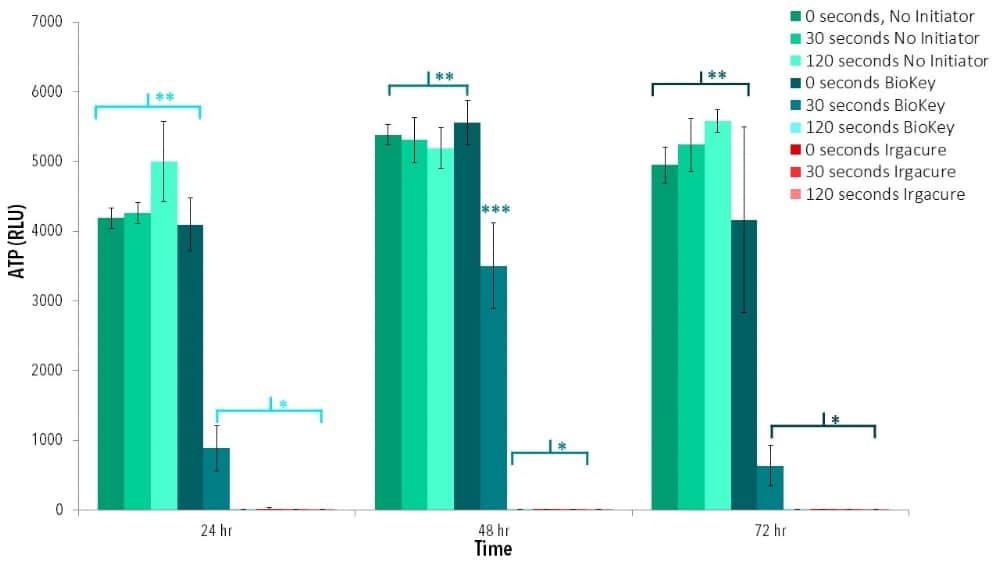
Crosslinking Process
Different methods of crosslinking may expose cells to harsh chemicals or processes. Additionally, varying the degree of crosslinking of a material may change inherent material properties, such as mechanical properties or material permeability. In turn, these altered material properties may affect cell viability.
Sample Thickness
How thick are your encapsulated thin films? Thicker than 0.2 mm? If so, this thickness may be the source of your decreased viability. See if you can adjust your thin film fabrication method to decrease this thickness. The good news with this viability issue is that bioprinting can also help with this! With bioprinted samples, you’ll have greater control over sample geometry and can even include microchannels in your structures to improve nutrient transport and waste export.
Bioprinted 3D Culture Variables
You finally have your 3D encapsulated pipetted controls from your encapsulation study and are now ready to begin bioprinting! Bioprinted 3D constructs have a few extra variables, detailed below, that can also affect viability. We suggest running a bioprint study to first create a bioprinted 3D control. Below are the variables, in addition to the ones above, to consider when you begin bioprinting.
Needle Type
Your needle shape and size can affect shear stress experienced by cells. Tapered needle tips decrease necessary pressure to print, decreasing shear stresses experienced by cells, while smaller needle diameters will increase the shear stress experienced by cells. Try setting up a 24-hour viability study to test the effects of different pressures and needle types on construct viability.
Print Pressure
Increased print pressure will increase the shear stress experienced by encapsulated cells. It is best to test a variety of print pressure and create 3D printed thin-film controls to monitor the effects of this variable on construct viability. Try setting up a 24-hour viability study to test the effects of different pressures and needle types on construct viability.
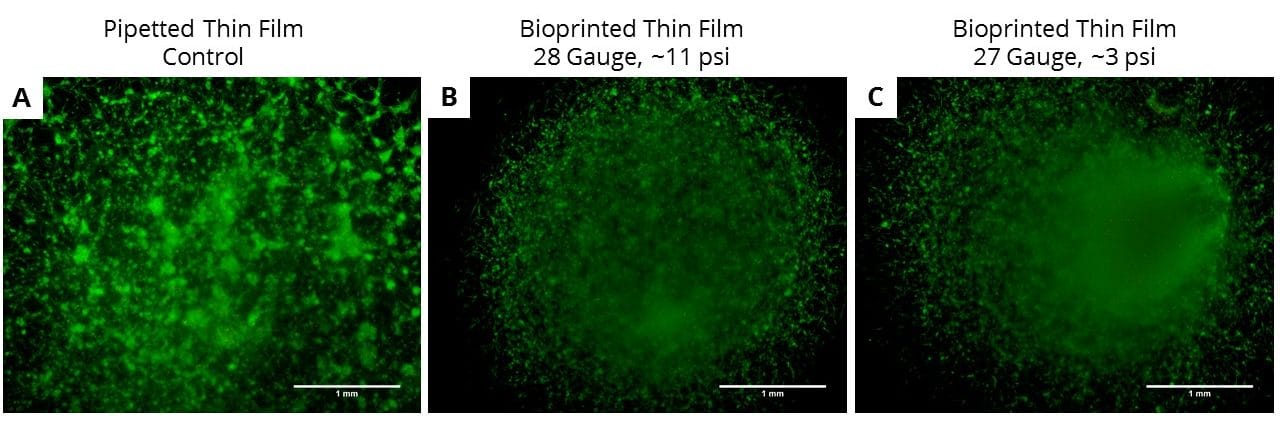
Print Time
Depending on the material, cell type, and print temperature, the time it takes to complete a print session may affect final construct viability. If you are experiencing varied viability in your constructs, this parameter may be the culprit. Keep track of how long your print sessions take and set up a study to determine maximum print time available for different bioink formulations.
Experiment Controls
After completing both encapsulation and bioprinting studies to characterize the variables above, we suggest using the following 3D controls in all future bioprinting studies. These controls will help you quickly pinpoint any potential issues with viability in your studies.
- 2D Control: We suggest a 2D control for each varying cell concentration, type, and ratio used in bioprinted samples.
- 3D Pipette Control: We suggest a 3D pipetted control (also referred to as thin films) for each different material, material concentration, crosslinking process, and cell type/concentration
- 3D Print Control: We suggest a 3D printed control (also referred to as thin films) for each of the different variables in 3D pipet controls as well as for each different pressure and needle type used during printing.
Learn more specifics about creating these 3D controls here.

