Overview
Corning® Matrigel® matrix is a hydrogel that is rich in extracellular matrix proteins. It is extracted from the Engelbreth-Holm-Swarm (EHS) mouse sarcoma, which is composed of laminin, collagen IV, heparan sulfate proteoglycans, entactin/nidogen, TGF-ß, epidermal growth factor, insulin-like growth factor, fibroblast growth factor, tissue plasminogen activator, and other growth factors. This protocol will walk you through a step-by-step process for 3D Bioprinting with Corning Matrigel.
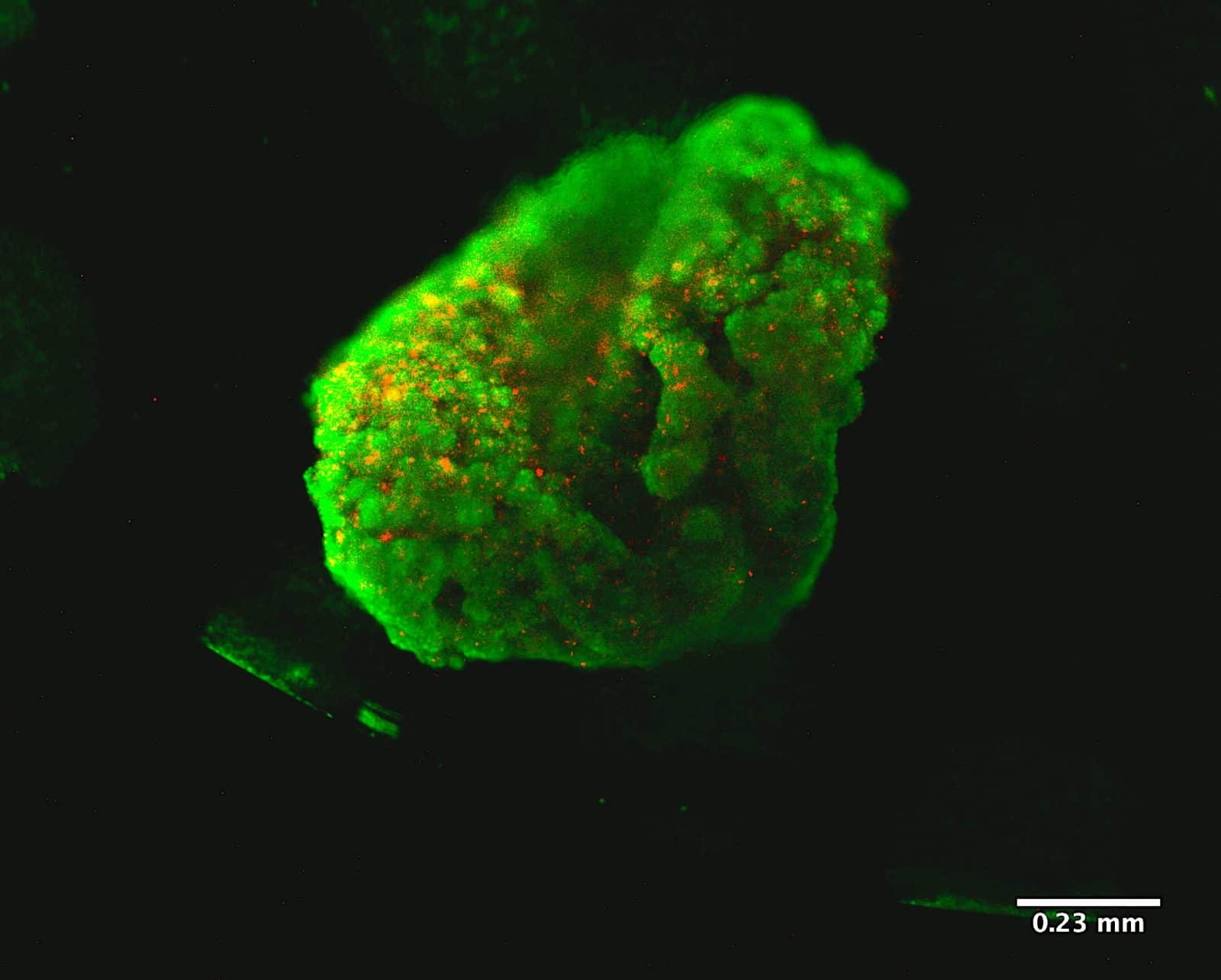
This hydrogel has been successfully used for several 3D culture and tissue engineering applications. Now, it can also be used on the Allevi Platform to 3D bioprint cancer spheroids with a variety of cell lines. Combining Matrigel matrix with Allevi 3D bioprinters can enable the automation of spheroid and organoid generation in a standardized and repeatable manner.
Storage and Handling
Corning Matrigel matrix should be stored at -20˚C. This protocol has been optimized for #354234 High Concentration Matrigel. Other Matrigel variations may require updated print parameters.
Materials
- Corning Matrigel basement membrane matrix (HC, #354234), 10 mL vial
- 1 x 15 mL Falcon® Centrifuge Tube
- 1 x Allevi 5 mL Syringe
- 1 x Syringe Cap
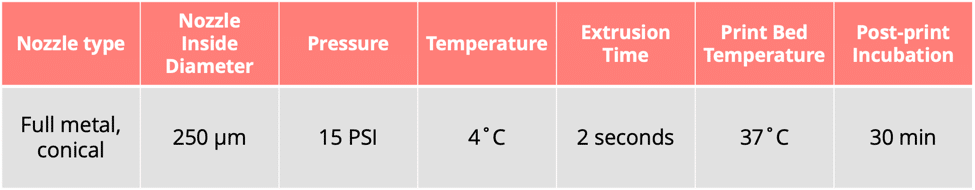
- 1 x Full Metal 250 µm Nozzle
- 1 x Costar® Multi-well Plate or Falcon® Petri Dish
- Ice Bucket
- Cells
- Cell media
- Incubator
Methods
- Remove Corning Matrigel matrix from -20˚C and thaw it in an ice bucket at 4˚C overnight;
- In a 15 mL Falcon® centrifuge tube, prepare a cell pellet containing the appropriate cell number needed for your experiment;
- NOTE: We recommend using a 2.5 million/mL cell concentration and preparing a total of 3 mL of cell-laden bioink. In this case, you would need to prepare a cell pellet containing 7.5 million cells.
- Cool the CORE™ printhead to 4˚C;
- Heat the Allevi print bed to 37˚C and insert your dish of choice (lid on) in order to allow it to reach your set temperature;
- With a pipette, add 3 mL (or desired volume) of Matrigel® to your cell pellet and thoroughly mix up and down to ensure cell homogeneity;
- NOTE: Perform this step with the tube inside the ice bucket to prevent premature crosslinking.
- Remove the plunger from a 5 mL syringe and place a syringe cap on the luer lock;
- Pipette your chilled Corning Matrigel matrix cell-laden solution into the syringe barrel, with the syringe cap facing down;
- Remove the black rubber stopper from the syringe plunger and place it in the opening of the syringe barrel;
- Flip the syringe barrel to face up, ensuring that the rubber stopper does not come off by holding it in place;
- Wait for the material to flow to the bottom, touching the rubber stopper;
- Remove the syringe cap;
- With the plunger, push the material up until it forms a meniscus over the Luer-Lock tip;
- NOTE: When performing this step, prevent the plunger from re-attaching to the stopper.
- If there are bubbles trapped in the syringe, tap the barrel to ensure that trapped air goes to the surface;
- Attach the 250 µm full metal nozzle to the syringe;
- Place the syringe in the CORE™ printhead, making sure to minimize Matrigel® exposure to room temperature to avoid premature crosslinking;
- Insert the recommended print parameters from the Print Settings section below to print your organoids;
- NOTE: To manipulate your droplet resolution (diameter and volume), check out the Printing Dots protocol;
- NOTE: The Matrigel® droplets will partially crosslink during your print session because of your heated surface, however, make sure to not let them sit on the surface for longer than 15 minutes. After 15 minutes, the droplets will start to dry out, which leads to lower cell viability.
- Place your Matrigel matrix droplet plate inside an incubator (37˚C, 95% relative humidity, 5% CO2) for 30 minutes;
- Add the appropriate amount of media for culture.
NOTE: For optimal print results, it is recommended to not further dilute Matrigel Matrix. If you would rather mix larger cell volumes with the ECM, a Cell-Bioink Mixing protocol is provided. It contains instructions on how to mix and prepare a cell-laden bioink by diluting it with cell-laden media.
NOTE: Check out our assay protocols to further analyze spheroid function.
Print Settings