Overview
Passaging is required when human mesenchymal stem cells have proliferated to confluences around 90%. This important step enables you to culture enough cells for your experiments, as well as maintain the health of your cell culture. This protocol will walk you through the process of passaging human mesenchymal stem cells, as well as keeping track of passage numbers to ensure that you are using passages that still represent good cell line functionality.
Materials
- Human Mesenchymal Stem Cell Flasks
- Mesenchymal Stem Cell Growth Medium (MSCGM™)
- Sterile tissue culture flasks or culture dishes of choice
- ReagentPack™ Subculture Reagents
- Trypsin/EDTA
- Trypsin Neutralizing Solution (TNS)
- HEPES Buffered Saline Solution (HBSS)
- Centrifuge Tubes
- Centrifuge
- Micropipettes
- Micropipette Tips
- Microscope
- Incubator (37˚C, 5% CO2, 90% humidity)
- Timer
- 70% Ethanol
- Kimwipes
- Vacuum Bottle
Methods
- Warm FGM-2™ medium and ReagentPack™ reagents to 37˚C in the water/bead bath;
- Spray them down with ethanol and place them inside a biosafety cabinet (BSC);
- Spray down incubator doors with ethanol and minimize breathing to diminish contamination risk;
- Remove cell culture flasks from incubator and place them under microscope to ensure there is no contamination;
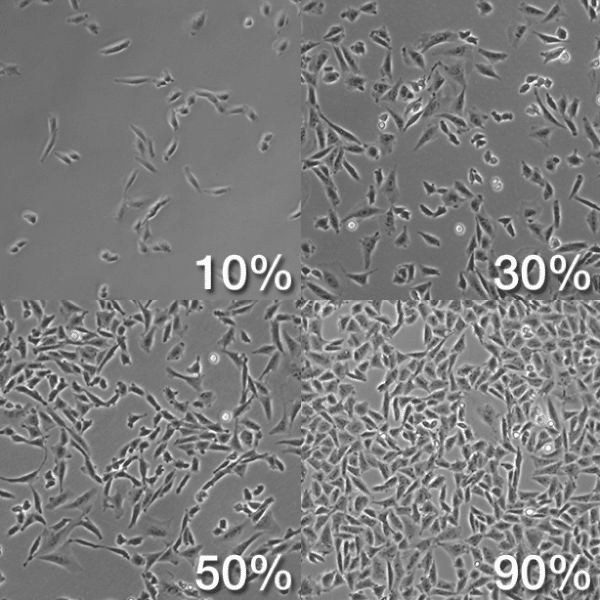
- Still, under the microscope, check for flask confluency by analyzing the ratio of surface area occupied by cells and surface area not occupied by cells. It is good practice to passage cells that are ~90% confluent to ensure a high yield;
https://biology.stackexchange.com/questions/29857/origin-of-term-confluency-in-cell-culture - Spray down flasks with ethanol, wipe them with Kimwipes and place them in BSC;
- Aspirate cell media from culture flasks, ensuring to avoid touching the bottom of the flask;
- Wash flask with HBSS, using the amount needed to cover your cell culture flask surface;
- Aspirate HBSS;
- Add trypsin/EDTA to the flask, using the amount needed to cover your cell culture flask surface (note this volume);
- Note: the recommended volume/flask surface area is 0.5 mL/10 cm2.
- Gently rock your flask and place it back into the incubator for 5 minutes;
- Check cells under the microscope to see if they have released from the bottom of the flask (are floating). If so, go to the next step. If not, spray the flask down with ethanol, wipe it with Kimwipes and place it into the incubator for another 5 minutes. Repeat this step until cells are released, ensuring to not exceed 15 minutes of trypsin/EDTA exposure, as it may be toxic to cells;
- Add the same volume of TNS to your flask as trypsin/EDTA in order to neutralize trypsin/EDTA activity;
- Pipette cell suspension into a centrifuge tube;
- Centrifuge at 600 X g for 5 minutes;
- Note: to convert from g to rpm, use the following formula: g = 1.12 x rotor radius (in mm) x (rpm/1000)2
- Spray down your centrifuge tube with ethanol and wipe it with Kimwipes before going back into the BSC;
- After centrifuging, you should be able to see a cell pellet at the bottom of the flask. Gently aspirate supernatant, ensuring to not disturb the pellet;
- Count your cells following this protocol;
- Find the number of flasks or dishes you will need to replate your cells by using the following formula;
- Number of flasks (nflasks) = Total number of cells/cell seeding density/Flask surface area (a).
- Note: human mesenchymal stem cell seeding density is 5,000-6,000 cells/cm2.
- You will need to add 1 mL of cell suspension to each flask, so add the remaining amount of media to your cell suspension tube (since you resuspended it in 1-5 mL for counting) to have a total volume of nflasks;
- Example: If you resuspended your cells in 1 mL and you are replating them in 5 flasks, add 4 mL to complete the suspension volume necessary to be able to add 1 mL of cell suspension per flask.
- Add 1 mL of cell suspension to each flask;
- Note: if you had a high yield of cells and want to replate them in fewer flasks than in the calculation above, use the remaining cell suspension and follow this protocol on how to freeze your remaining cells.
- Find the amount of cell media you will need to culture your cells by using the formula below;
- Volume of culture media = 0.2 mL * flask surface area in cm2.
- Add the volume of media you found on the previous step to each flask;
- Label your flasks. It is good practice to include the following information;
- Your initials and date of passage (e.g.: if your initials are YN, and the date is MM/DD/YYYY, label it as YNYYYMMDD)
- Cell line (hMSCs) and passage number (if your flasks were Pn, they will now be Pn+1).
- Note: these cells can only be passaged until P5. After that, functionality and viability may be compromised.
- Feed cells every three to four days, following this protocol;
- Passage cells when they reach 90% confluence, following this protocol.