
Introduction
Poly(ethyelene glycol) diacrylate, or PEGDA, is one of the most common synthetic hydrogels used for cell encapsulation. The advantages of this biomaterial include biocompatibility, hydrophilicity and the ability to chemically tailor the material (1-3). This study tests the viability of bioprinted PEGDA (MW 3400), Percoll, and lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) with an Allevi printer.
See Also
- Bioprinted Alginate Viability
- Bioprinted Collagen Viability
- Bioprinted GelMA and LAP Viability
- LAP and Irgacure Viability with GelMA
- PLGA Viability Report
Results
Crosslinking Viability
First, a variety of crosslinking times were tested to determine if there would be any variations in viability. Thin films of 20% PEGDA solutions with 0.5% LAP concentrations were crosslinked for periods of 2, 5 and 10 minutes and cultured up to 7 days. No significant differences in viability were found at any of the crosslinking times.
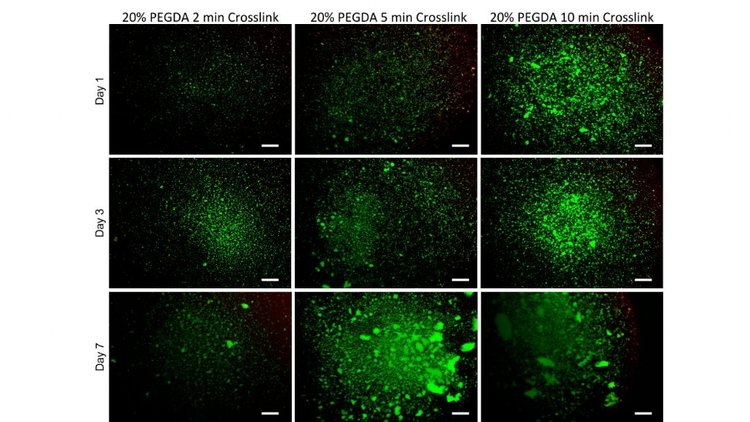
Cellular Distribution
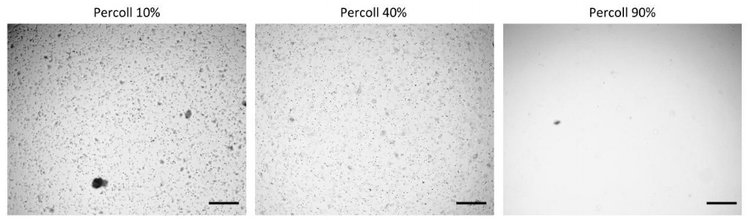
To improve cellular distribution and uniformity during printing, Percoll was added to PEGDA(3). A centrifugation test was completed with 10, 40 and 90% Percoll. Next, cells were cultured with formulations of 20% PEGDA and either 10, 40 or 90% Percoll for 24 hours and analyzed via LIVE/DEAD staining. No significant differences in viability were found among the varying solutions.
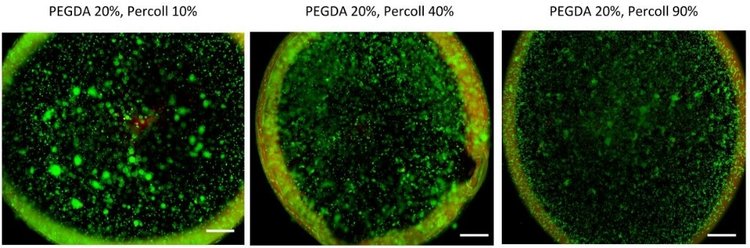
Bioprinted Viability
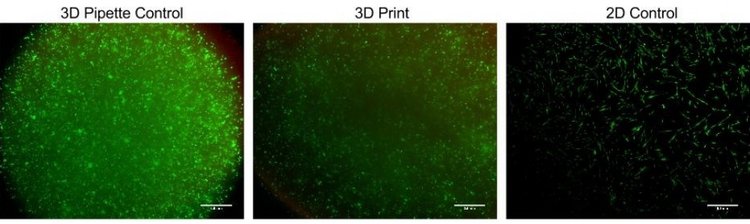
Thin films were created via pipetting and bioprinting and compared after 1 and 7 days of culture. Live/Dead images demonstrate similar viability between thin films and bioprinted PEGDA. After 7 days of culture, images of bioprinted and pipetted thin films were taken on a confocal microscope. 3D renderings further confirmed resolution and viability of printed structures.
While some specific 3D structures, such as 200 μm thick cylinders with a 200 µm diameter, were attempted by infilling pluronic support structures, variations in pressure during printing did not allow for consistent, accurate fabrication of these structures.
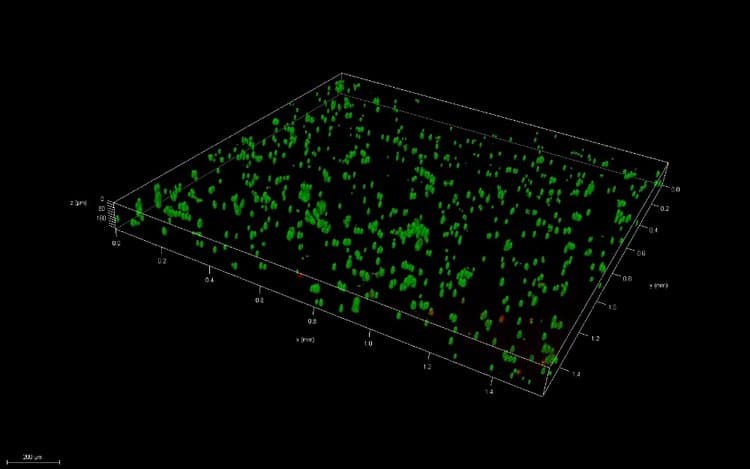
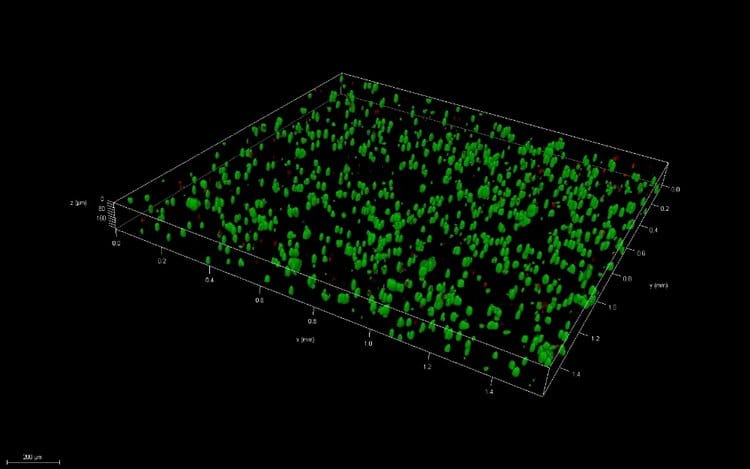
Conclusions
Allevi offers a commercially available platform with reagents, hardware, and software that allow users to create viable 3D structures with complex, reproducible geometries. Through the Allevi platform, this study demonstrates the fabrication of viable bioprinted cultures composed of cell-laden PEGDA, Percoll, and LAP.
Due to low viscosity, consistency in PEGDA printed structures is difficult to achieve even with temporary support materials such as pluronic. It is suggested to combine this reagent with a more viscous biomaterial for improved printability.
Methods
Cell Culture
Primary Human Neonatal Dermal Fibroblasts (HNDFs) obtained from ATCC were cultured in monolayer cultures at 37 °C and 5% of CO2 using Dulbecco’s Modified Eagle Medium (Corning) supplemented with 10% fetal bovine serum (Hyclone) and 1% penicillin-streptomycin-amphotericin (Corning). Passage numbers under 12 were used.
Cell Distribution Test
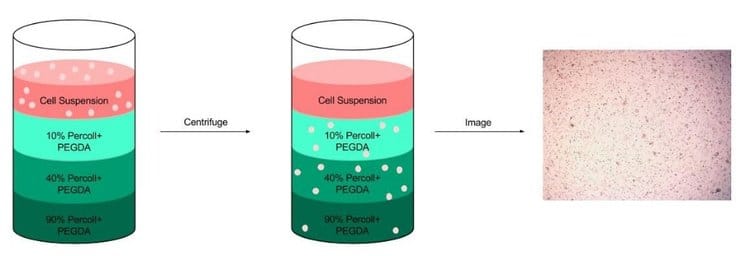
To minimize cell settling during bioprinting, Percoll (Sigma-Aldrich) was added to PEGDA/LAP solutions(3). A gradient ranging from 10 – 90% Percoll prepared with 20% PEGDA solutions was created in a 15 ml centrifuge tube (3 layers of 1 ml each) (3). A suspension of HNDFs (1 ml of 1 x 106 cells) was pipetted on top of the gradient, then the tubes were centrifuged at 200 x g for 5 min. After, cell number in each fraction was analyzed through brightfield imaging (3).
Thin Film Fabrication
To create thin films, glass coverslips were treated according to the protocol. Then, LAP was dissolved in cell culture media under stirring and heat (60˚C). Next PEGDA was mixed in each solution. After the mixture was completely dissolved and homogeneous, it was sterile filtered (0.45 μm) (Millipore) as suggested in the protocol provided by Allevi.
Once solutions were filtered, each was heated to 37°C and mixed with Percoll for final concentrations of 20% (w/v) PEGDA, 0.5% (w/v) LAP and either 10, 40 or 90% (v/v) Percoll. After mixing with Percoll, HNDFs were added at a 5 million/ml concentration. This mixture was then pipetted onto treated glass at 10 μl. Next, the solution was exposed to blue light (405 nm) at 10 mW/cm² for 2, 4, 5 or 10 minutes. For all bioprinting studies, crosslink times of 4 minutes were used. The thin films, stuck on the glass, were then placed into 24 well plates (Corning).
Bioprinted Thin Film Fabrication
G-code files for developed to extrude material for 3 seconds, as well as to infill a pluronic support disc. Pressures of 3.4-4.8 psi were used to print PEGDA solutions, along with tapered metal 150 µm diameter needles. Thin films were printed onto treated glass slides, then crosslinked for 4 min with blue light (405 nm) at 10 mW/cm². All samples were bioprinted with an Allevi 2.
Sample Analyses
To assess cell viability, A LIVE/DEAD kit (Life Technologies) was used. Images were taken on a Nikon TE300 Inverted Fluorescent Microscope or a Leica TCS SP5 II Scanning Laser Confocal Microscope.
References
- B. D. Fairbanks et. al, “Photoinitiated Polymerization of PEG-diacrylate with lithium phenyl-2,4,6-trimethylbenzoylphosphinate: polymerization rate and cytocompatibility,” Biomaterials, vol. 30, no. 35, pp. 6702-6707, Dec 2009.
- Chan V et al. “Three-dimensional photopatterning of hydrogels using stereolithography for long-term cell encapstulation.” Lab Chip. 2010(10) 2062-70.
- Lin, Hang et al. “Application of Visible Light-based Projection Stereolithography for Live Cell-Scaffold Fabrication with Designed Architecture.” Biomaterials 2013(34). pp. 331- 39.

